Questions: dissolved in a liter of water. acid strong or weak? species present at (10^-6 mol/L) or greater when dissolved in water --- --- --- (mathrmH2 mathrmCO3) weak (mathrmH2 mathrm~S) weak (mathrmHClO2) weak HI strong
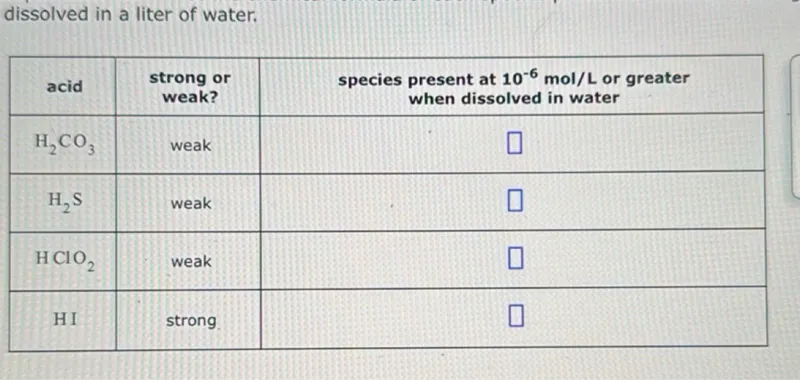
Transcript text: dissolved in a liter of water.
\begin{tabular}{|c|c|cc|}
\hline acid & \begin{tabular}{c}
strong or \\
weak?
\end{tabular} & \begin{tabular}{c}
species present at $\mathbf{1 0}^{-6} \mathbf{~ m o l / L}$ or greater \\
when dissolved in water
\end{tabular} \\
\hline $\mathrm{H}_{2} \mathrm{CO}_{3}$ & weak & $\square$ \\
\hline $\mathrm{H}_{2} \mathrm{~S}$ & weak & $\square$ \\
\hline $\mathrm{HClO}_{2}$ & weak & $\square$ \\
\hline HI & strong & $\square$ \\
\hline
\end{tabular}





