Questions: The compound magnesium chloride is a strong electrolyte. Write the reaction when solid magnesium chloride is put into water. Include states of matter in your answer. MgCl2(s) → Mg^2+(aq) + 2Cl^-(aq)
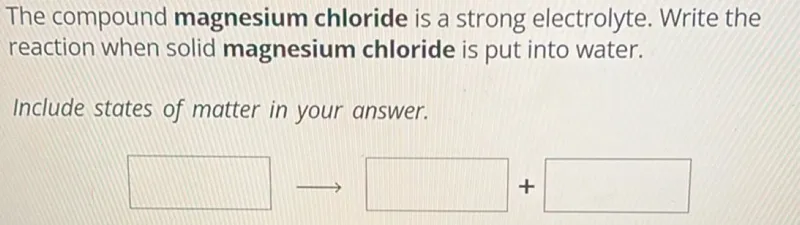
Transcript text: The compound magnesium chloride is a strong electrolyte. Write the reaction when solid magnesium chloride is put into water.
Include states of matter in your answer.
$\square$ $\longrightarrow$ $\square$ $+$ $\square$





