Questions: Determine the rate of reaction for the following chemical reaction, based on the information given: 2 H2O2(aq) → 2 H2O(l) + O2(g) Reaction started with 1.4 M H2O2 and ended with 0.6 M after 0.8 minutes. Make your answer's precision to two decimal places. Include the correct units in your answer for full credit.
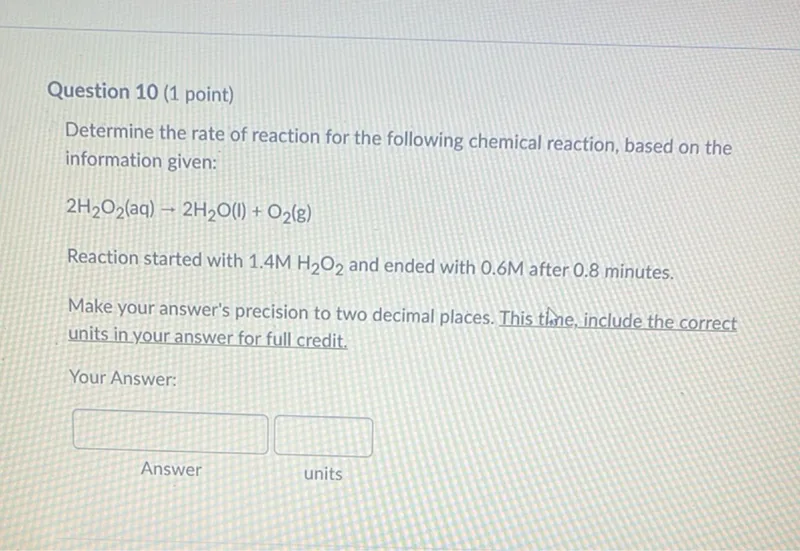
Transcript text: Determine the rate of reaction for the following chemical reaction, based on the information given:
\[
2 \mathrm{H}_{2} \mathrm{O}_{2}(\mathrm{aq}) \rightarrow 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})+\mathrm{O}_{2}(\mathrm{~g})
\]
Reaction started with $1.4 \mathrm{M} \mathrm{H}_{2} \mathrm{O}_{2}$ and ended with 0.6 M after 0.8 minutes.
Make your answer's precision to two decimal places. This thine, include the correct units in your answer for full credit.





