Questions: In chemical reactions, heat is converted into chemical energy (the potential energy stored in chemical bonds) or vice versa. Bond energy is the energy required to takes energy to break a bond, bond energies are always positive. Conversely, energy is released when bonds are formed. Thus, the enthalpy change for a reaction can be approximated from ΔHran=Σ(ΔH breaking )+Σ(ΔH forming ) where H represents bond energies for the breaking (positive bond energy) or forming (negative bond energy) of a bond and Hmas represents the overall enthalpy for the reaction. Use the table to answer questions about bond energies. Bond Bond energy (kJ / mol) C-C 347 C=C 611 C-H 414 C-O 360 C=OinCO 799 O-O 142 O=O 498 H-O 464 Calculate the approximate enthalpy change, ΔHmax, for the combustion of one mole of methane as shown in the balanced chemical equation: CH4+2 O2 → 2 H2O+CO2 Use the values you calculated in Parts A, B, C, and D, keeping in mind the stoichiometric coefficients Express your answer to three significant figures and include the appropriate units.
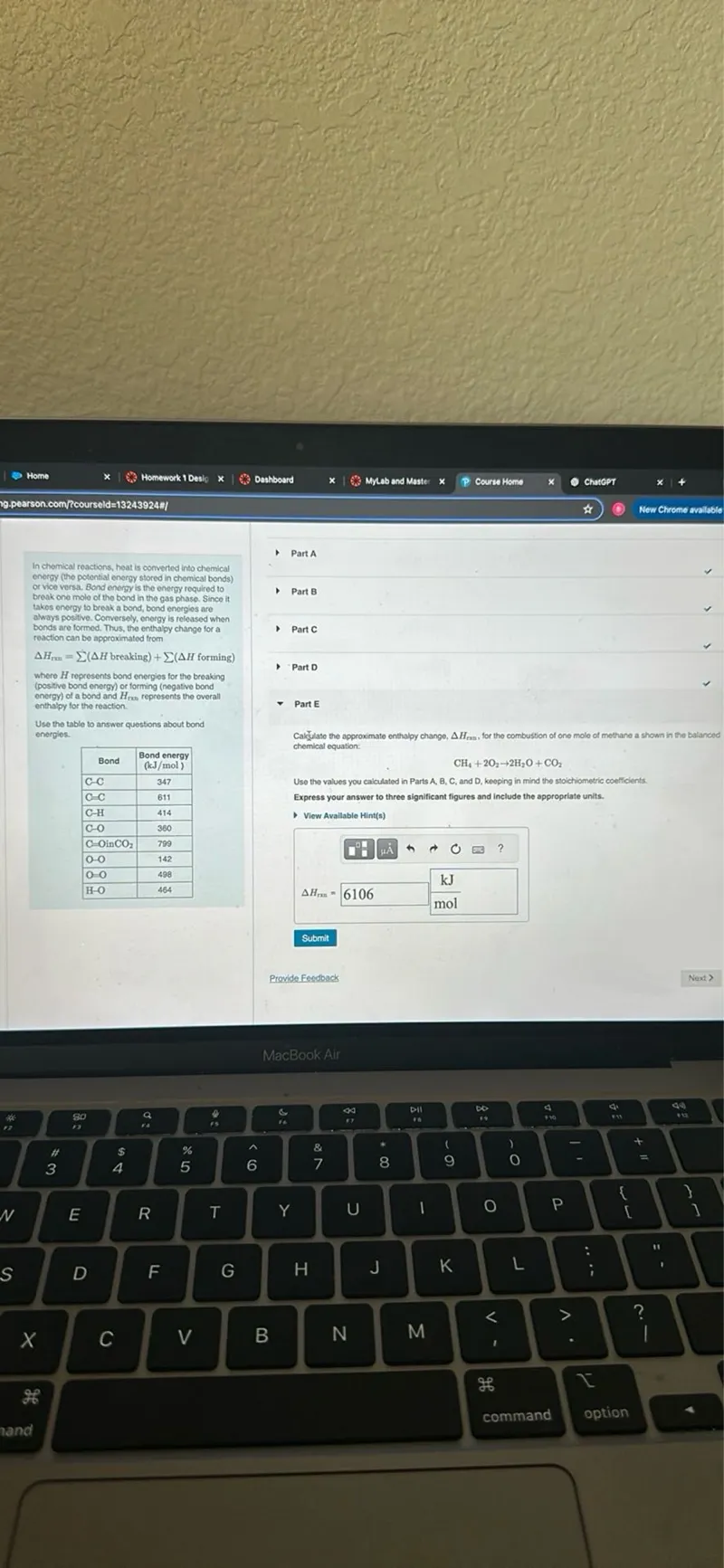
Transcript text: In chemical reactions, heat is converted into chemical energy (the potential energy stored in chemical bonds) or vice versa. Bond energy is the energy required to takes energy to break a bond, bond energies are always positive. Conversely, energy is released when bonds are formed. Thus, the enthalpy change for a reaction can be approximated from
$\Delta H_{\mathrm{ran}}=\sum(\Delta H$ breaking $)+\sum(\Delta H$ forming $)$ where $H$ represents bond energies for the breaking (positive bond energy) or forming (negative bond energy) of a bond and $H_{m a s}$ represents the overall enthalpy for the reaction. Use the table to answer questions about bond energies.
\begin{tabular}{|l|c|}
\hline \multicolumn{1}{|c|}{ Bond } & \begin{tabular}{c}
Bond energy \\
$(\mathrm{kJ} / \mathrm{mol})$
\end{tabular} \\
\hline $\mathrm{C}-\mathrm{C}$ & 347 \\
\hline $\mathrm{C}=\mathrm{C}$ & 611 \\
\hline $\mathrm{C}-\mathrm{H}$ & 414 \\
\hline $\mathrm{C}-\mathrm{O}$ & 360 \\
\hline $\mathrm{C}=\mathrm{OinCO}$ & 799 \\
\hline $\mathrm{O}-\mathrm{O}$ & 142 \\
\hline $\mathrm{O}=\mathrm{O}$ & 498 \\
\hline $\mathrm{H}-\mathrm{O}$ & 464 \\
\hline
\end{tabular}
Calculate the approximate enthalpy change, $\Delta H_{\mathrm{max}}$, for the combustion of one mole of methane as shown in the balanced chemical equation:
\[
\mathrm{CH}_{4}+2 \mathrm{O}_{2} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}
\]
Use the values you calculated in Parts A, B, C, and D, keeping in mind the stoichiometric coefficients Express your answer to three significant figures and include the appropriate units.





