Questions: Identify the Electron Configuration for Aluminum (Al) 1s^2 2s^2 2p^6 3s^2 3p^1 1s^2 2s^2 2p^6 3s^2 4p^1 1t^2 2p^2 2p^6 3f^2 3p^3
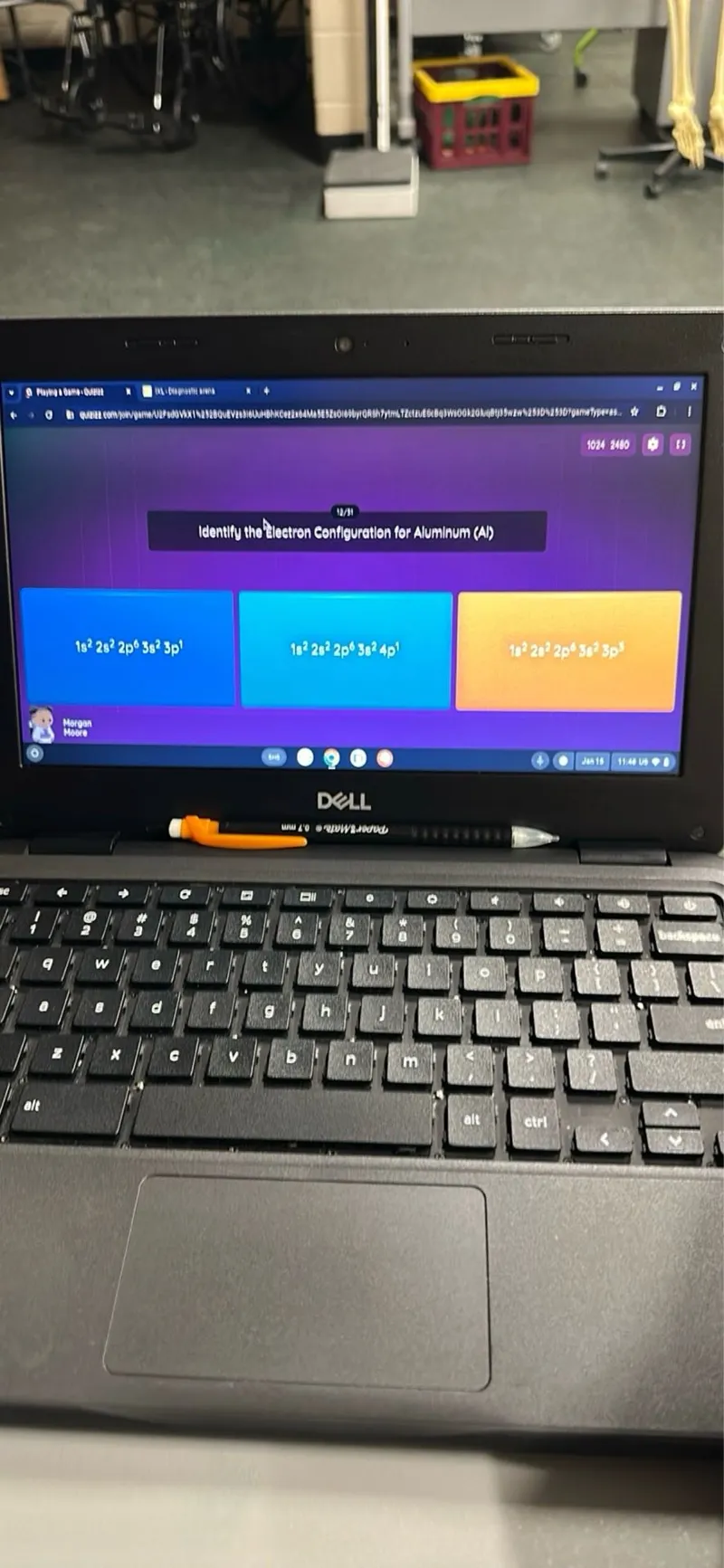
Transcript text: Identify the Electron Configuration for Aluminum (Al)
$1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{1}$
$1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 4 p^{1}$
$1 t^{2} 2 p^{2} 2 p^{6} 3 f^{2} 3 p^{3}$





