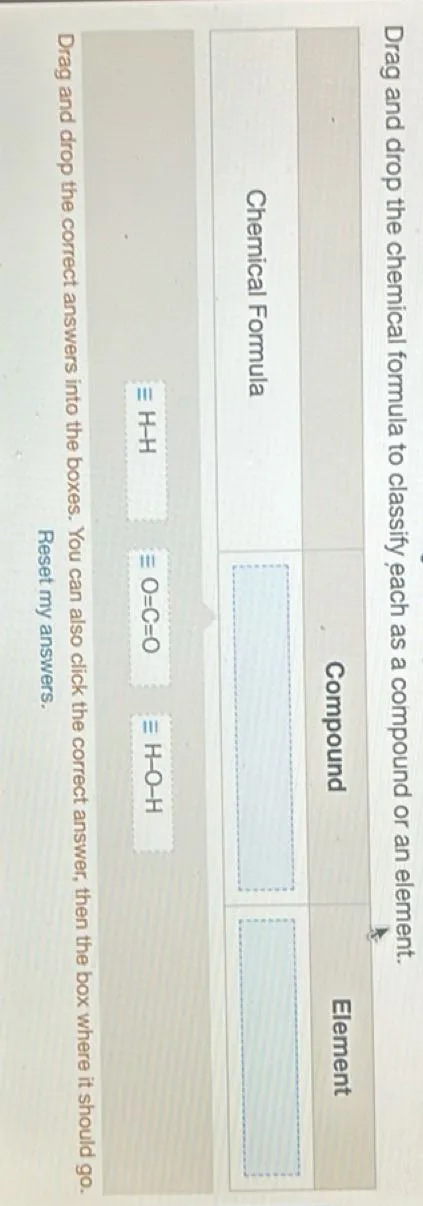
Classify the chemical formula as a compound or an element.
Understanding the difference between compounds and elements.
An element is a pure substance consisting of only one type of atom, represented by a single chemical symbol (e.g., \( \text{O}_2 \), \( \text{N}_2 \)). A compound is a substance formed when two or more different types of atoms are chemically bonded together, represented by a chemical formula with multiple elements (e.g., \( \text{H}_2\text{O} \), \( \text{CO}_2 \)).
Classifying the given chemical formula.
To classify a chemical formula, identify if it contains only one type of element or multiple elements. If it contains only one type of element, it is an element. If it contains more than one type of element, it is a compound.
\(\boxed{\text{Classify each chemical formula based on the above criteria.}}\)
To classify a chemical formula as a compound or an element, determine if it consists of only one type of atom (element) or multiple types of atoms (compound).