Questions: What is the oxidation level in the molecule below? Type in the number: 1,2,3, etc.
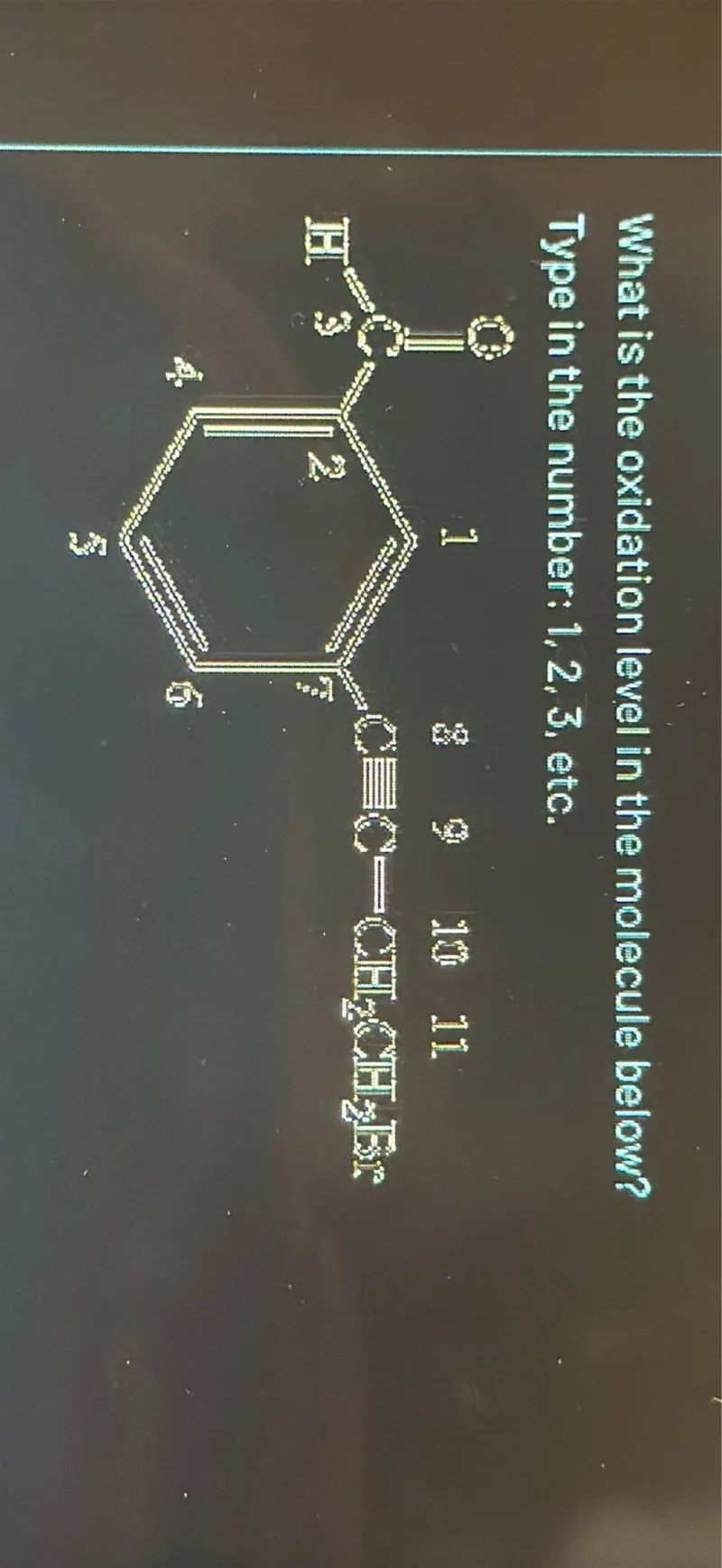
Transcript text: What is the oxidation level in the molecule below?
Type in the number: $1,2,3$, etc.





