Questions: 9) C8H18 + O2 → CO2 + H2O 10) FeCl3 + NaOH → Fe(OH)3 + NaCl 11) P + O2 → P2O5
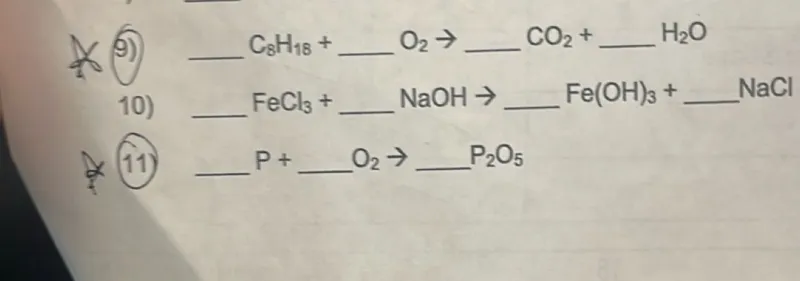
Transcript text: 9) $\mathrm{C}_{8} \mathrm{H}_{18}+$ $\qquad$ $\mathrm{O}_{2} \rightarrow$ $\qquad$ $\mathrm{CO}_{2}+$ $\qquad$ $\mathrm{H}_{2} \mathrm{O}$
10) $\qquad$ $\mathrm{FeCl}_{3}+$ $\qquad$ $\mathrm{NaOH} \rightarrow$ $\qquad$ $\mathrm{Fe}(\mathrm{OH})_{3}+$ $\qquad$ NaCl
11) $\qquad$ $P+$ $\qquad$ $\mathrm{O}_{2} \rightarrow$ $\qquad$ $\mathrm{P}_{2} \mathrm{O}_{5}$





