Questions: Fill in the missing information: symbol atom or ion? check all that apply number of protons number of electrons ----------------------------------------------------------------------------------- neutral atom cation anion 48 neutral atom cation anion 17 18 Mn^3+ neutral atom cation anion
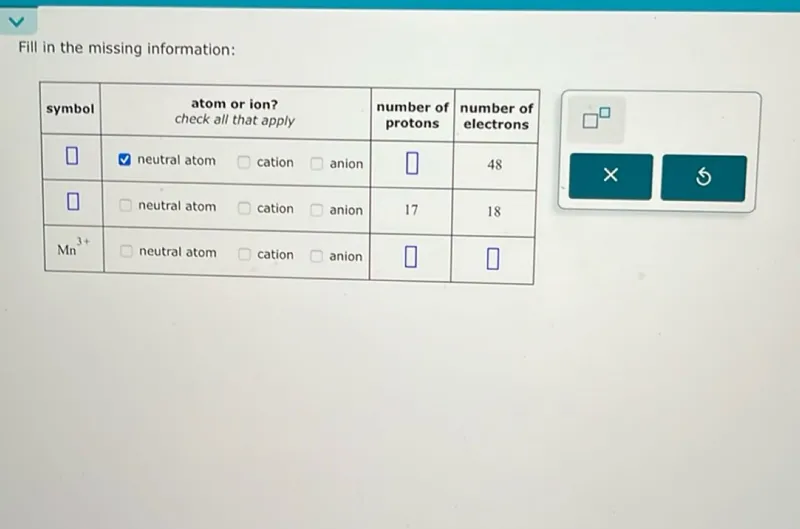
Transcript text: Fill in the missing information:
\begin{tabular}{|c|cc|c|c|c|}
\hline symbol & \begin{tabular}{c}
atom or ion? \\
check all that apply
\end{tabular} & \begin{tabular}{c}
number of \\
protons
\end{tabular} & \begin{tabular}{c}
number of \\
electrons
\end{tabular} \\
\hline$\square$ & $\square$ neutral atom & $\square$ cation & $\square$ anion & $\square$ & 48 \\
\hline$\square$ & $\square$ neutral atom & $\square$ cation & $\square$ anion & 17 & 18 \\
\hline $\mathrm{Mn}^{3+}$ & $\square$ & neutral atom & $\square$ & cation & $\square$ anion \\
\hline
\end{tabular}





