Questions: Part C Ionic compounds are formed from ionic bonds, whereby an electron is transferred from the metal cation to the nonmetal anion. Ions form solid lattices of ions. Covalent compounds form solids through the attraction of two covalent molecules. Since the attraction between two covalent molecules is weak compared to the ionic bonds holding an ionic compound together, ionic compounds tend to have higher melting points. Which of the following compounds has the highest boiling point? Br2 NaBr HBr BrF
Transcript text: Part C
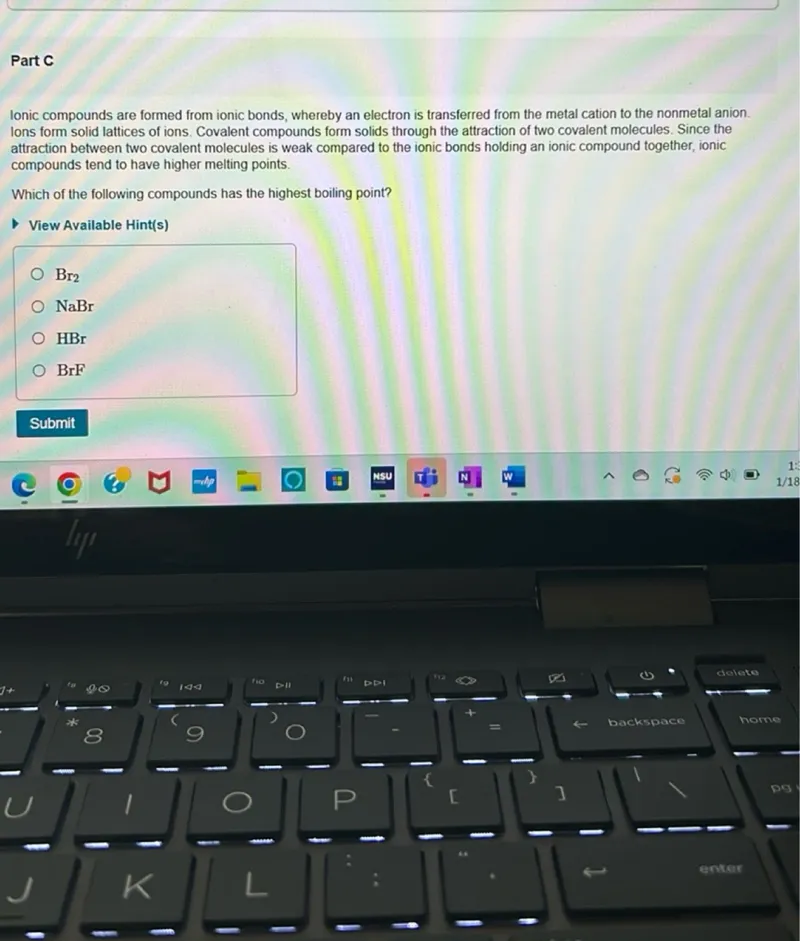
lonic compounds are formed from ionic bonds, whereby an electron is transferred from the metal cation to the nonmetal anion. Ions form solid lattices of ions. Covalent compounds form solids through the attraction of two covalent molecules. Since the attraction between two covalent molecules is weak compared to the ionic bonds holding an ionic compound together, ionic compounds tend to have higher melting points.
Which of the following compounds has the highest boiling point?
View Available Hint(s)
$\mathrm{Br}_{2}$
NaBr
HBr
BrF
Submit





