Questions: Aluminum has an electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^1. Carbon has an electron configuration of 1s^2 2s^2. How is the electron configuration of Aluminum different from Carbon? Select the two correct answers. (1 point) Carbon has 2 electrons in its p orbital. Carbon has 3 electrons in its p orbital. Aluminum has 4 electrons in its porbital. Carbon has 6 electrons in its p orbital. Aluminum has 6 electrons in its porbital.
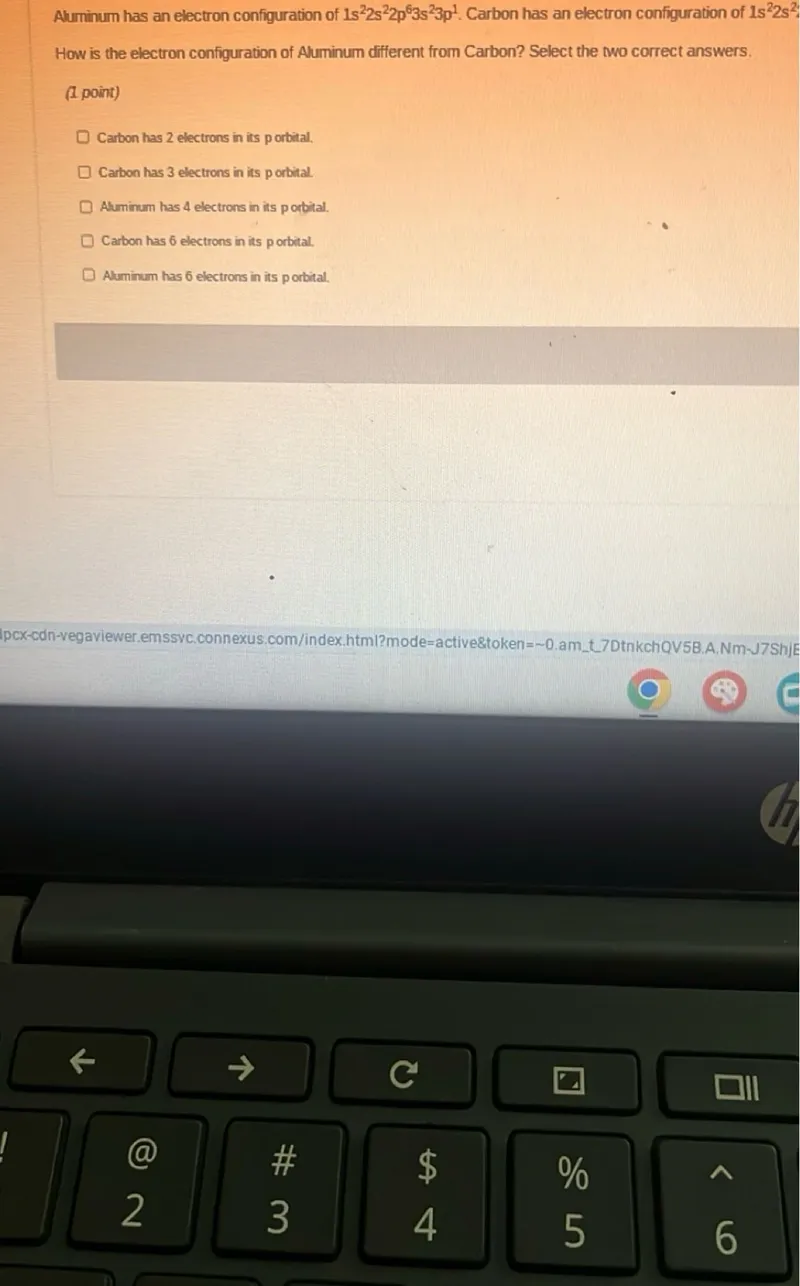
Transcript text: Aluminum has an electron configuration of $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{1}$. Carbon has an electron configuration of $1 s^{2} 2 s^{2}$
How is the electron configuration of Aluminum different from Carbon? Select the two correct answers.
(1 point)
Carbon has 2 electrons in its p orbital.
Carbon has 3 electrons in its p orbital.
Aluminum has 4 electrons in its porbital.
Carbon has 6 electrons in its p orbital.
Aluminum has 6 electrons in its porbital.





