Questions: Midterm Review Units 1-4 Question 5 The volume of a gas is 4.00 liters at 293 K and constant pressure. For the volume of the gas to become 3.00 liters, the Kelvin temperature must be equal to: A. 3.00×293 B. 293/3.00 C. 4.00×293 D. 3.00×4.00/293 E. 293/4.00×3.00 Submit Answer
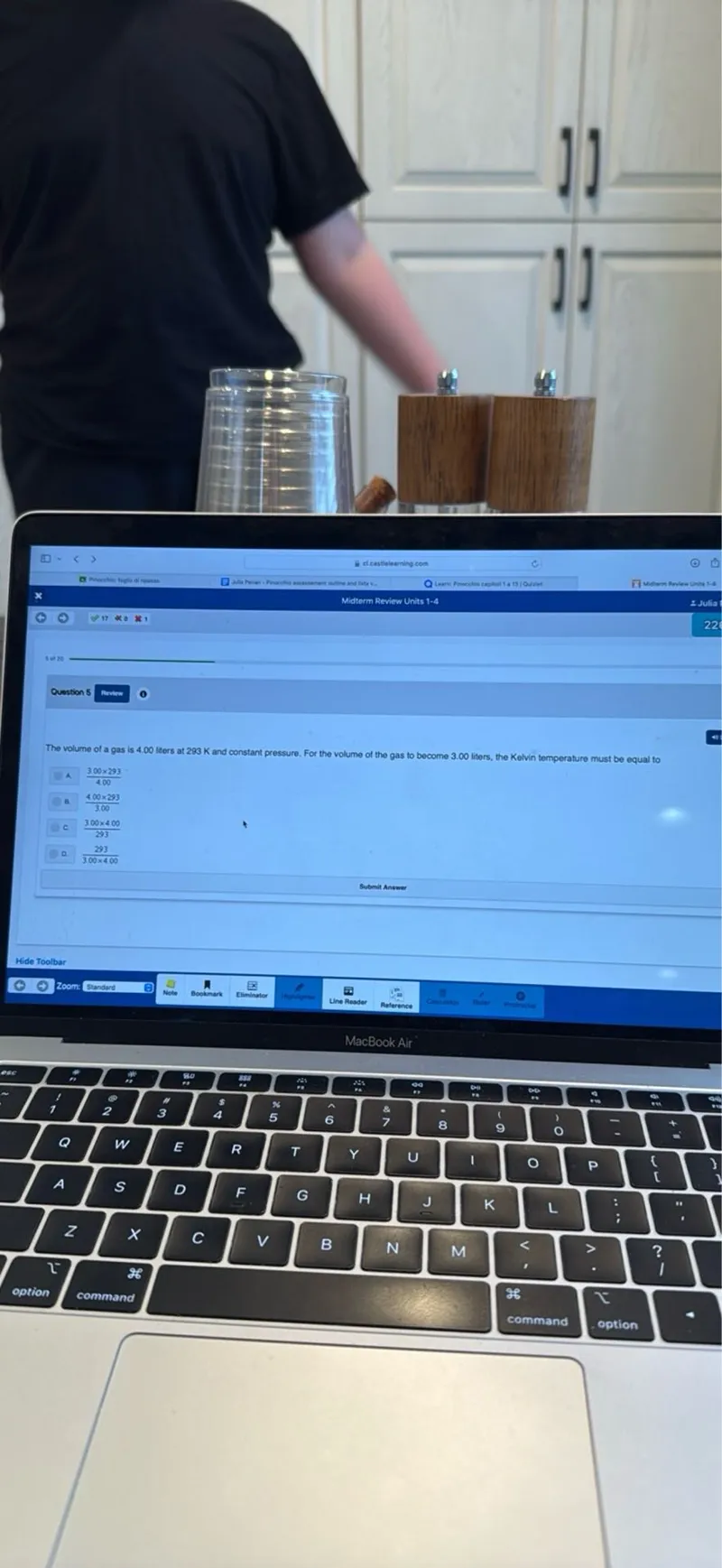
Transcript text: Midterm Review Units 1-4
Question 5
The volume of a gas is 4.00 liters at 293 K and constant pressure. For the volume of the gas to become 3.00 liters, the Kelvin temperature must be equal to:
A. 3.00×293
B. 293/3.00
C. 4.00×293
D. 3.00×4.00/293
E. 293/4.00×3.00
Submit Answer





