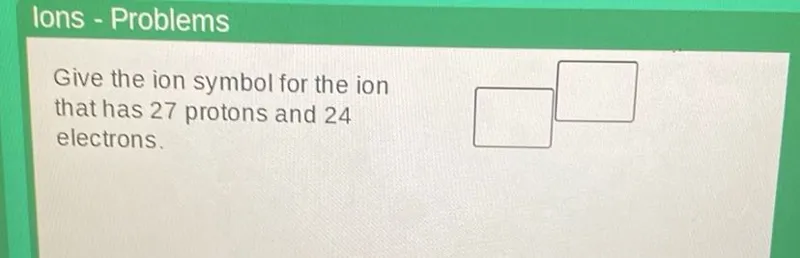
Questions: Ions - Problems Give the ion symbol for the ion that has 27 protons and 24 electrons.
Transcript text: Ions - Problems
Give the ion symbol for the ion that has 27 protons and 24 electrons.





