Questions: What is the molarity of a solution prepared by dissolving 4 grams of NaOH in 100 mL of water? 5.00 M 0.50 M 1.00 M 0.01 M
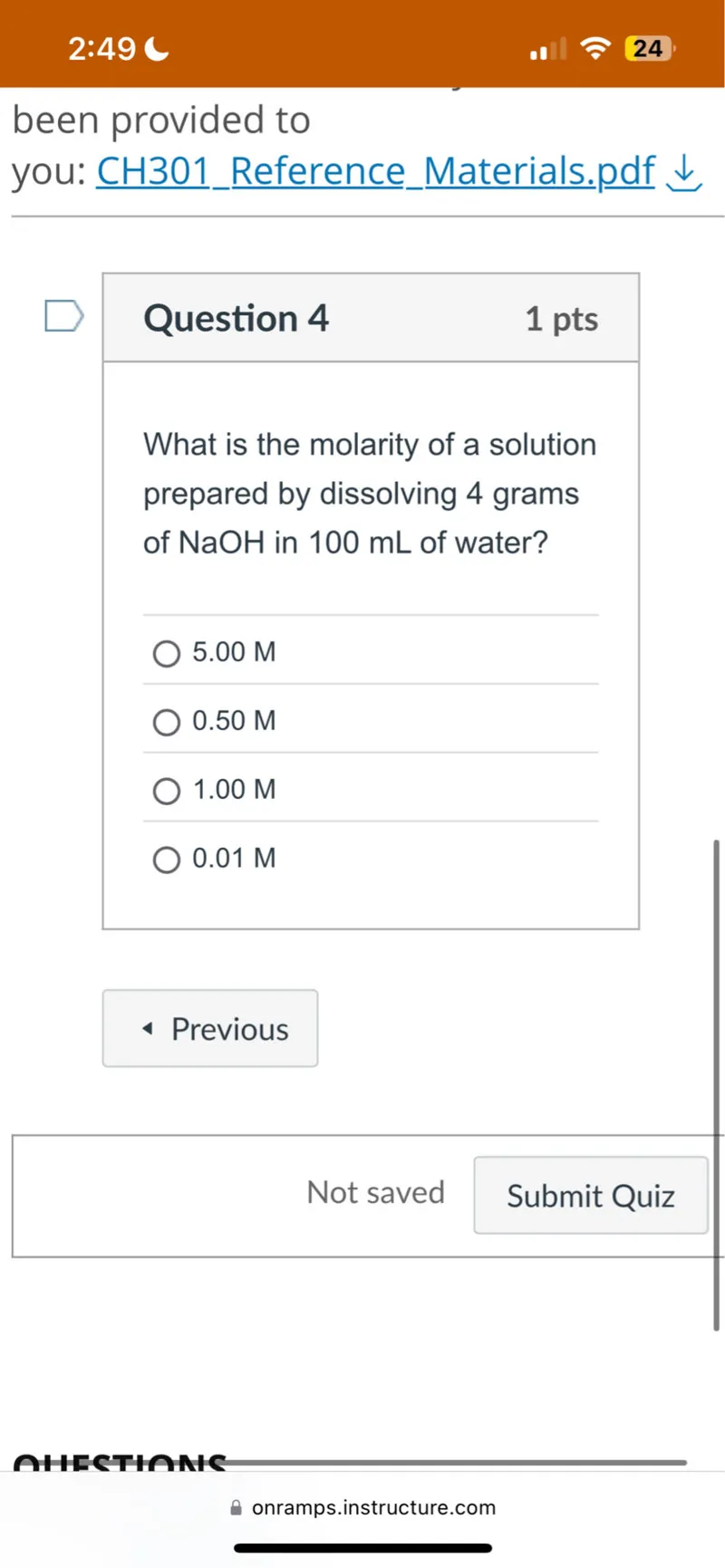
Transcript text: What is the molarity of a solution prepared by dissolving 4 grams of NaOH in 100 mL of water?
5.00 M
0.50 M
1.00 M
0.01 M





