Questions: Which compounds would you expect to contain one or more covalent bonds? Compounds - CBr4 - S8 - H2O - S2F10 - N2O3
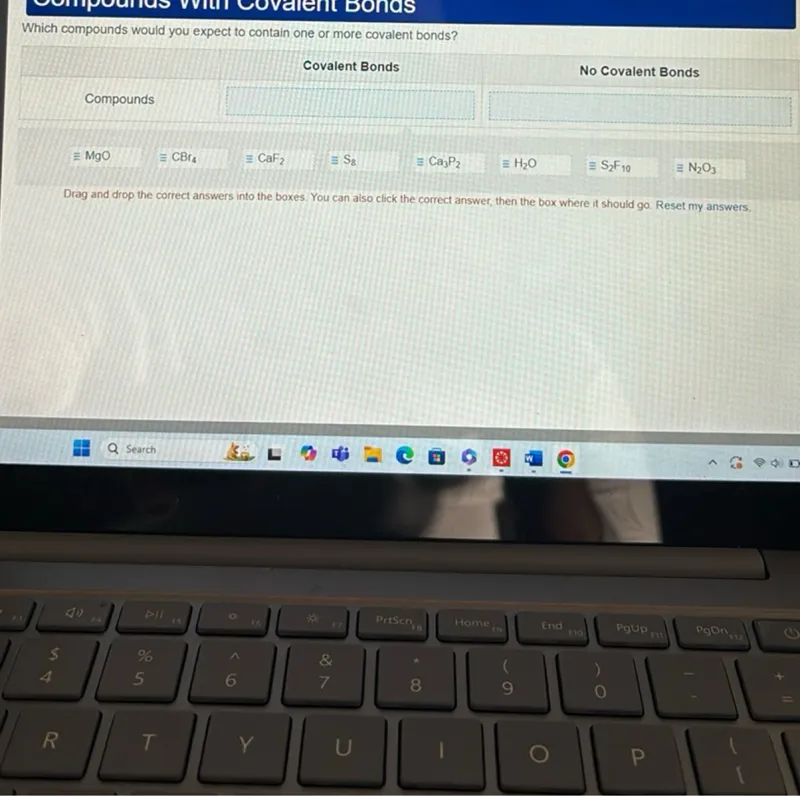
Transcript text: Which compounds would you expect to contain one or more covalent bonds?
Compounds
- CBr4
- S8
- H2O
- S2F10
- N2O3





