Questions: Complete each Lewis structure by adding lone pairs to give each atom an octet. If no lone pairs are needed, check "There are no missing lone pairs." For each indicated atom in the Lewis structure, identify its shape. Part 1 of 2 There are no missing lone pairs. Shape around the bold red C atom: Tetrahedral
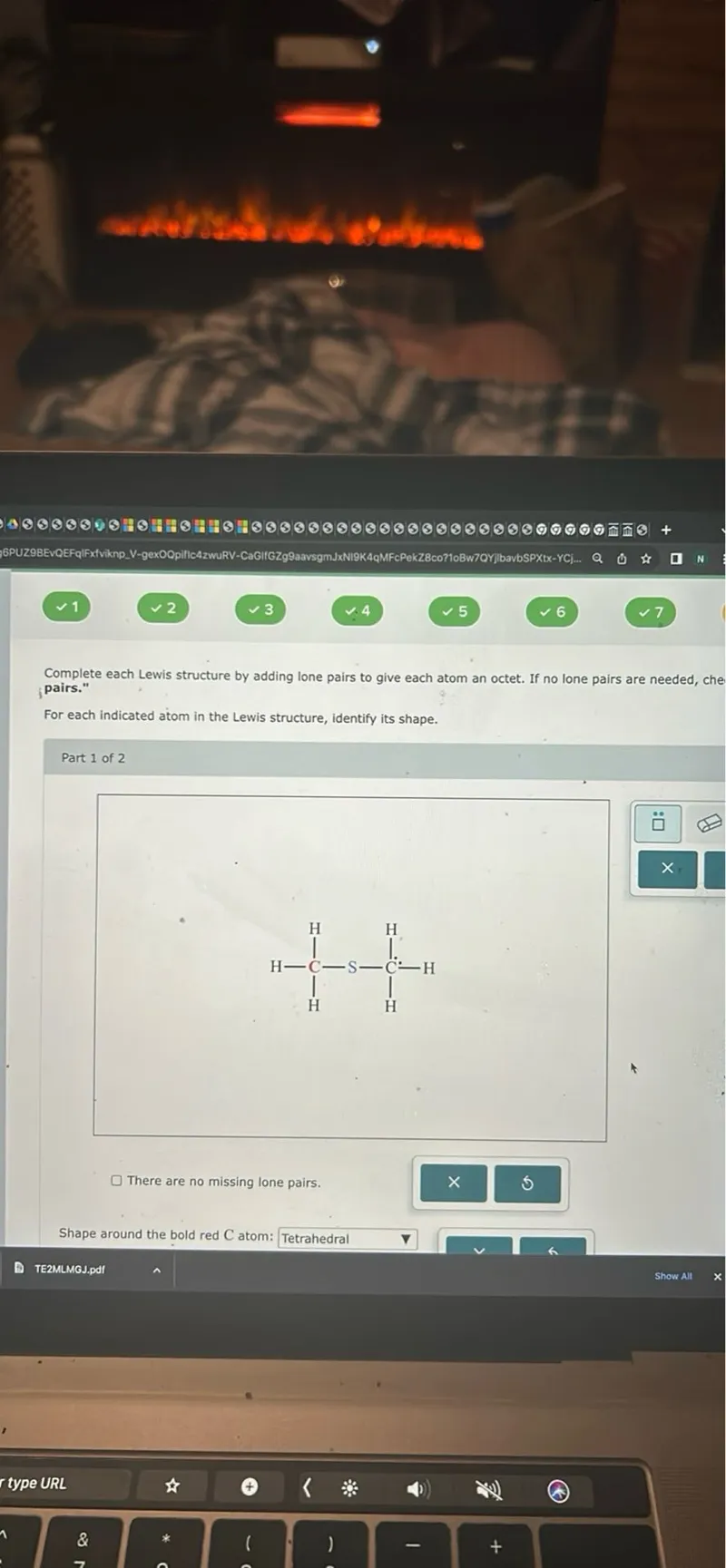
Transcript text: Complete each Lewis structure by adding lone pairs to give each atom an octet. If no lone pairs are needed, check "There are no missing lone pairs."
For each indicated atom in the Lewis structure, identify its shape.
Part 1 of 2
There are no missing lone pairs.
Shape around the bold red C atom: Tetrahedral





